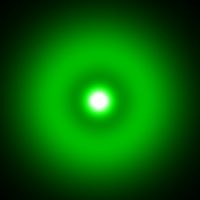
Methane CH4 molecule. Intensity is proportional to
∫ |Ψ(r)|2dz.
Use your fingers or mouse to control the model.
Use "zoom" (e.g. mouse wheel) to adjust intensity.
Canvas is matched to your browser window.
Valence bond theory
Valence bond theory proposes that covalent bonds form when the atomic orbitals
of two atoms overlap. The combined, overlapping orbitals, contain a total of
two electrons with opposed spins.
Atomic (Hydrogen) orbitals
Hydrogen wave functions are
Ψ1s ~ 2 e -r,
Ψ2s ~ (2 - r) e -r/2,
Ψ2px ~ x e -r/2.
σ bonds
Sigma (σ) bonds arise from the 'end-on' overlap between adjacent orbitals.
This leads to a region of high electron density along the inter-nuclear axis.
Hybrid orbitals
Hybridization of atomic orbitals provides for molecular
shapes that cannot be accommodated by using s, p, and d
orbitals.
This hybridization allows carbon to form four rather than two covalent
bonds and to orient them so as to minimize the e- pair repulsions
(i.e., tetrahedrally).
Comments
1. The fragment shader shader-fs-calc for every (x, y) point
(pixel) renders integrated intensity into 64x64 texture. Then
this texture is mapped on the whole canvas. For complex molecule orbitals are
rendered in sequence.
2. Laguerre integration formula for integral of the type
∫o∞ f(z)e-zdz is used
(25.4.45 "Handbook of Mathematical Functions"
under the direction of M.Abramowitz and I.Stegun)
∫o∞ g(z)dz =
∑i wi e zi g(zi )
where wi e zi, zi
are tabulated values for i = 1, 9. Integration may be not very accurate
(but simple, fast and funny :)
References
[1] Lecture 18
- Theories of Covalent Bonding by Warren Gallagher
[2] Atomic
orbital viewer by Andy Brummer
Open Source molecule viewers
updated 3 May 2012